Methods
Integrative Neuroscience
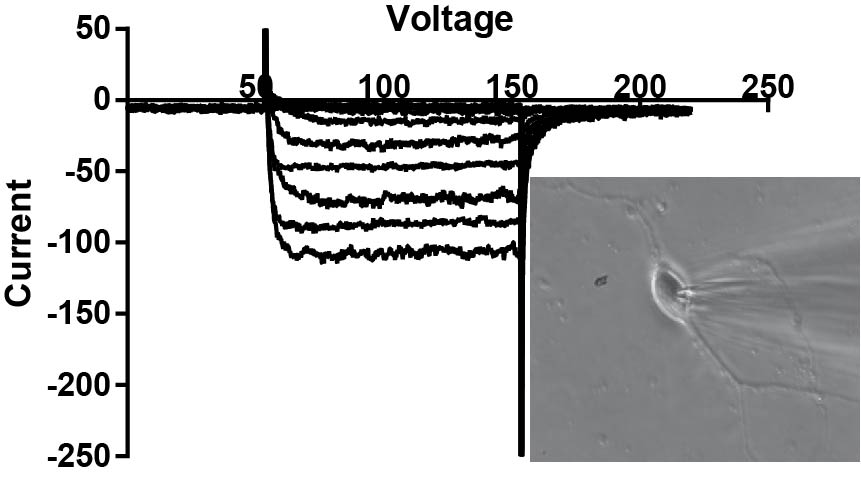
Slice electrophysiology
What we study
Brain slice electrophysiology and imaging is a primary method in the lab. There are three slice "rigs" in the lab, each on an Olympus microscope backbone using Molecular Devices/Axon amplifiers. Each have flourescent capabilities for recording of labeled pathways. One rig has a 3i Yokagawa confocal microscope for probing intracellular mechansims and release of neurotransmitters via flourescence. We have examined nTS, PVN and spical cord pathways.
Intrinsic plasticity
Gating of sensory afferent input
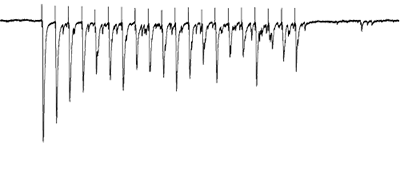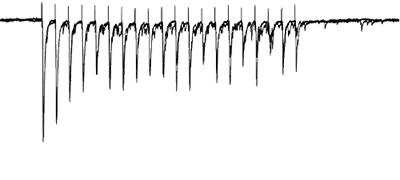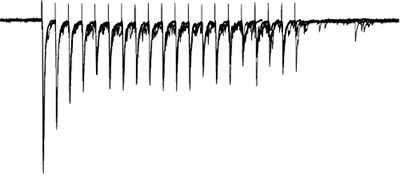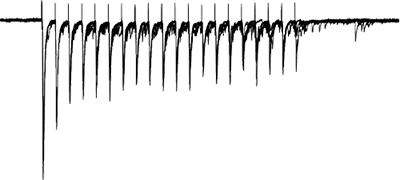
Afferents stimulated at 20Hz, note the adapatation
Activation of specific pathways
Opto- and chemogentic manipulation of circuits
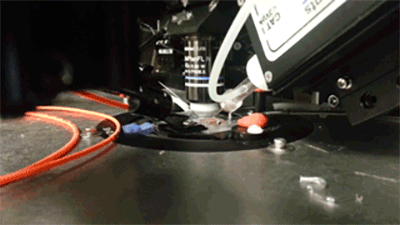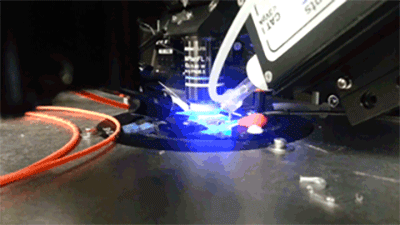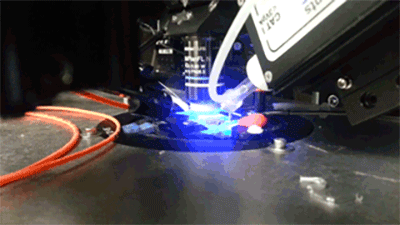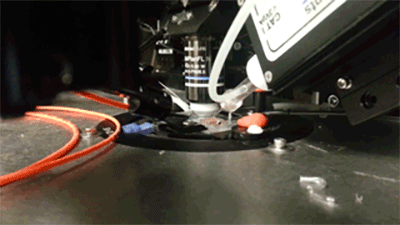
Prior expression of channelerhodopsin and activation via 470 nm light
Imaging of sensory circuits
Terminal Calcium imaging
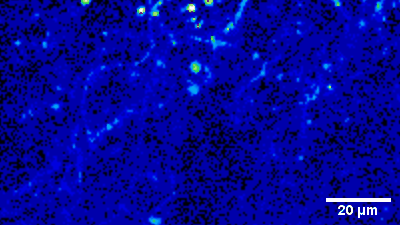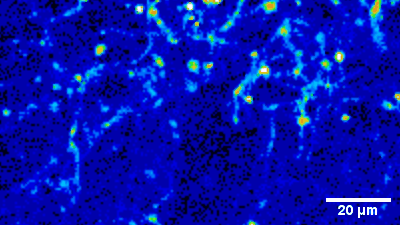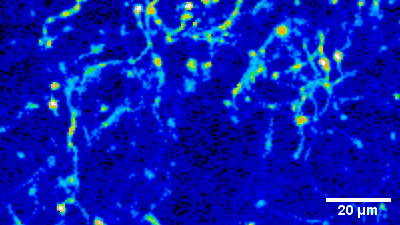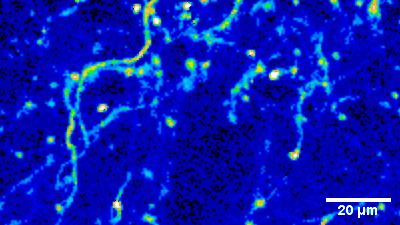
Prior expression of GCamP 6M in nodose ganglia and electrical activation